For healthcare professionals

Introducing Mydrane®

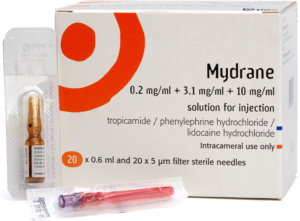
- Mydrane is an intracameral (IC) injection containing tropicamide 0.2 mg/ml (0.02%), phenylephrine 3.1 mg/ml (0.31%) and lidocaine 10 mg/ml (1%)
- One dose of 0.2 ml contains 0.04 mg of tropicamide, 0.62 mg of phenylephrine hydrochloride and 2 mg of lidocaine hydrochloride
- Tropicamide and phenylephrine work synergistically as mydriatics while addition of lidocaine improves patients comfort over topical anaesthesia alone1
Mydrane is indicated for cataract surgery to obtain mydriasis and intraocular anaesthesia during the procedure, offering an alternative method of mydriasis without the need for pre-operative mydriatic drops. The two mydriatics provide a rapid, stable mydriasis for the duration of the cataract surgery.1,2
Use of Mydrane may support cataract service efficiency, providing:4
- Speed
- 95% of pupil dilation within 30 seconds1,2
- Stability
- significantly more stable mydriasis than topical mydriatic drops3
- Comfort
- patient comfort was significantly superior during lens implantation1
Key Features
- The fastest method of Mydriasis for cataract surgery1,2
- Significantly more stable mydriasis than with topical mydriatic drops3
- Contains lidocaine for patient comfort
- Removal of preoperative Mydriatic regime may contribute to cataract service efficiency
- Significantly lower systemic passage of phenylephrine than with mydriatic drops5
- Comparable to mydriatic drops with respect to endothelial cell loss and corneal thickness5
How to use
At the start of the surgical procedure, slowly inject 0.2 ml of Mydrane intracamerally in one injection into the anterior chamber of the eye through the side port or principal port.
Pupil dilation is not required prior to surgery however, Mydrane should only be used in patients who have demonstrated, at pre-operative assessment, a satisfactory pupil dilation with topical mydriatic therapy.
The safety and efficacy of Mydrane in children aged 0 to 18 years have not been established.
For single eye use only.
Use immediately after first opening of the ampoule.
Pack contents
One box contains 20 sterile ampoules and 20 sterile filter needles.
Pricing
Hospital only product
NHS Price: £119.95 for one box containing 20 ampoules and 20 filter needles
Available through MPW only
References
- Labetoulle M et al. Br J Ophthalmol 2016;100:976-985.
- Internal report on phase II clinical study on small pupils. Data summary in Clinical Overview (LT2380-PII-09/12).
- Mydrane Summary of Product Characteristics.
- Patel J. et al. J Ophthalmic Surg 2017;1:1-6.
- Internal report on Phase III clinical study (LT2380-PIII-05/10).
Page last updated: 20th December 2024